Overview: Despite the insanely high growth in sales worldwide, thermal safety concerns are the most intolerable pain point in lithium-ion batteries and are the subject of research for technological advancements. This article will provide an overview of it.

Author’s illustration of a Lithium ion battery pack after suffering from thermal runaway
Lithium-ion Batteries
Lithium-ion batteries (LIBs) are the subject of extensive research and are used in all-electric vehicle applications. LIBs have been used a lot as the main power source for electric vehicles (EVs) because they have a high energy density, do not have a memory effect, last a long time, and are easy to design. Li-ion batteries' electrochemical properties depend on the cathode's lithium metal oxide and the anode's graphite.
This technology has over 90% efficiency. Some commercial products have a round-trip efficiency of over 95%, a high energy density (90-190 Wh/kg), and a lifecycle of a battery of up to 10,000 cycles, depending on the li-ion chemistry. This technology has a 190 Wh per kilogram energy density. Despite contributing to deterioration, cell temperature affects the battery’s lifetime.
Lithium-ion batteries, widely used in electronics, have become the dominant EV technology. Although expensive, this technology is great for grid-connected applications. A LIB cell has two parts: a positive electrode and a negative electrode, separated by a separator. During charging and discharging, lithium ions move back and forth between two electrodes, while electrons are forced through external circuitry to source and sink power, as shown in Fig. 1. The high volumetric and gravimetric energy densities are both made possible by the high cell voltage and capacity, which come from the careful choice of materials and careful design of the battery.
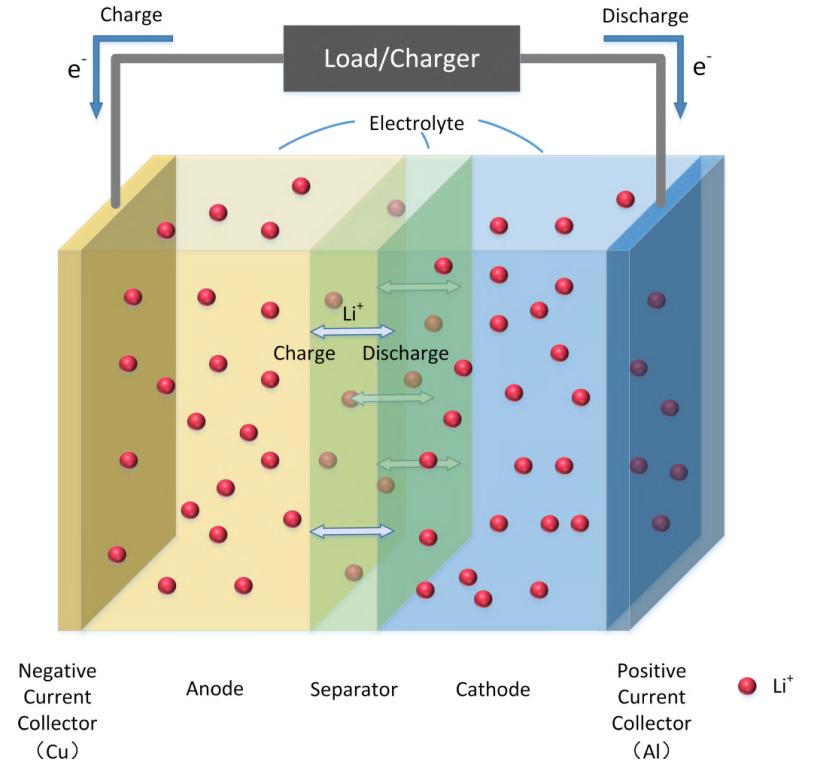
Fig. 1. Schematic of the Lithium-ion battery. Source: IEEE Access
Causes of Thermal Runaway
To meet the demands for power and energy, an EV battery pack typically consists of hundreds of thousands of cells connected in parallel and/or series. This substantially increases the energy that is stored inside battery systems, which results in expanded destructive effects when a serious safety issue arises, as illustrated in Fig. 2. On the other hand, LIBs in a vehicle are constantly subjected to difficult working conditions like vibration and shock, and under extremely abusive circumstances, they may also experience overcharging, overheating, short circuits, collisions, or nail penetration. This increases the chance that onboard battery systems will experience thermal runaway. Thermal safety concerns have, in general, become a barrier to the widespread use and market penetration of EVs.
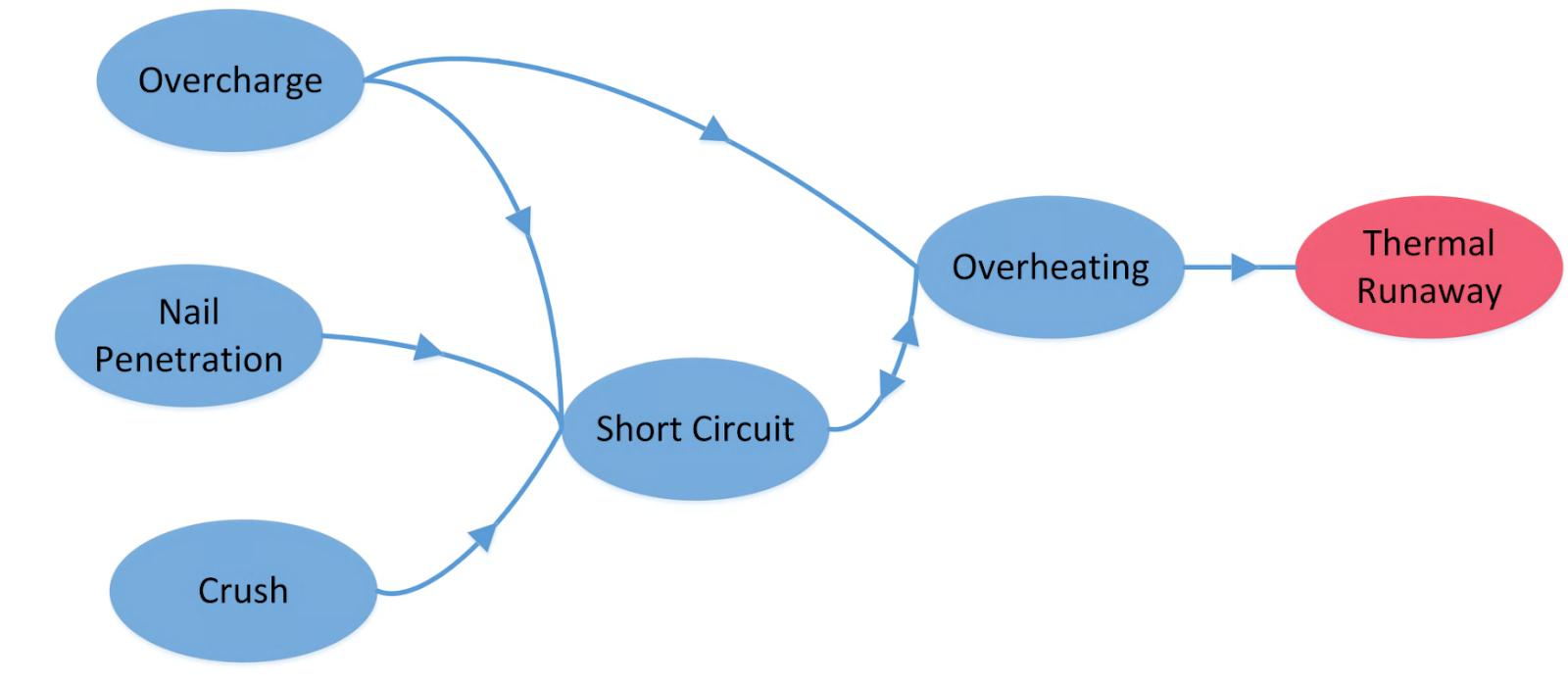
Fig. 2. Relationships among different abuse conditions of lithium-ion batteries. Source: IEEE Access
Typically, mechanical, electrical, or heat abuses can cause thermal runaway. The most common types of mechanical abuse are penetration and collision, which can result in internal short circuits in battery cells or bus-bar short circuits. Overcharging an electrical device can result in an internal short circuit due to lithium plating and set off a chain of subsequent exothermic side reactions. High environmental temperatures and/or ineffective thermal control are typically the causes of heat abuse.
The performance of the battery can be increased while heat generation is decreased by appropriately raising the LIB's working temperature. However, extremely high temperatures may result in separator shrinkage and exothermic decomposition of electrode and electrolyte materials, which can result in internal short circuits. Abuse behavior never happens individually in real-world circumstances; it always happens in succession. The aforementioned abusive behaviors eventually develop into an intense and quick heat-generation process that causes smoke, fire, and even an explosion.
In a typical electrochemical system, LIBs operate according to the electrochemical reaction, mass and charge transfer, and energy balance principles. Electrochemical reaction rates vary with time, temperature, and current distributions in a battery cell, making heat generation a complex process. Thermal runaway accidents, from the perspective of safety, mean upsetting the dynamic equilibrium between heat generation and accumulation inside battery cells and heat dissipation with their surroundings.
Therefore, it is important to shed light on the mechanism of heat generation and to present the most recent research on the modeling and testing of the intricate thermal process. Heat generation and thermal modeling strategies for LIBs are some possible solutions.
Thermal Modeling
For many years, researchers have introduced and studied the thermal modeling of LIBs. The underlying electrochemical reactions inside battery cells are now better understood thanks to the development of multi-dimensional and electrochemical-thermal-coupled modeling techniques. Gradually, control-oriented modeling emerges as a research hotspot with the potential to support battery management systems (BMSs).
Experiments for Thermal Behavior Investigation
Even though physical-based thermal modeling can give insights and theoretical explanations about how heat is made inside a LIB cell, experimental methods are still needed for parameterization and validation of the model. Calorimetry techniques are a basic way to measure temperature or heat flow within a certain range of temperatures. The heat balance equation can be used to find out how much heat an object produces. Two calorimetry methods that are often used are ARC (Accelerating Rate Calorimetry) and IHCC (Isothermal Heat Conduction Calorimetry).
ARC-Based Experiments
ARC is a tool or method for figuring out how much heat something makes. By creating an adiabatic environment, it can measure how much heat an object gives off. For a small number of chemicals, traditional ARCs are good and easy to use. But large-scale ARCs are being made and used to test LIBs, especially those with a large format.
IHCC-Based Experiments
IHCC has an isothermal test environment so that the temperature of the battery being tested stays the same. The isothermal boundary is always made by a sink with a fixed temperature that is in direct contact with the battery cells or by a chamber with a single temperature control point that lets heat move between the chamber well and the battery cells.
IHCCs come in two types that are often used in experiments. One is micro-calorimetry, which uses a sample cell and a reference cell placed in separate heat sinks. The other is a one-heat-sink design, which uses the Peltier effect or a power compensation method to control the sink temperature and measure the heat flux.
In short, calorimetry is the main tool used to study the thermal behavior of LIBs through experiments. Calorimeters can be made and set up in a certain way so that they can test large commercial, automotive batteries. It's also important to note that ARC has become a popular tool for thermal runaway tests on batteries because it works by self-heating and can be used in extreme test situations.
Summarizing with key points:
Some of the takeaways from the article are as follows:
- Due to their high energy density, lack of memory effect, longevity, and ease of design, LIBs have frequently been used as the primary power source for EVs.
- The LIBs in a vehicle, on the other hand, are constantly exposed to challenging working conditions like vibration and shock, and under extremely abusive conditions, they may also experience overcharge, overheat, short circuit, collision, or nail penetration.
- Thermal runaway is frequently brought on by mechanical, electrical, or heat abuses. Penetration and collision are the two most frequent forms of mechanical abuse.
- The most frequent form of electrical abuse is overcharging a device. The usual causes of heat abuse are high ambient temperatures and/or ineffective thermal control.
- The mechanism of heat production must be clarified, and the most recent findings on the modeling and testing of the complex thermal process must be introduced.
- Within a certain temperature range, calorimetry techniques are a simple way to measure temperature or heat flow. ARC and IHCC are two examples.
- By creating an adiabatic environment, ARC is a tool or method that can gauge how much heat an object emits.
- IHCCs have two varieties. One is micro-calorimetry, which makes use of reference and sample cells that are placed in different heat sinks.
- The other is a single-heat-sink design that measures heat flux and controls sink temperature using the Peltier effect or a power compensation technique.
- ARC's self-heating and extreme test capabilities make it a popular tool for thermal runaway tests on batteries.
This blog post is part of a full research article from IEEE Access.






